ADD ANTIMICROBIAL CAPABILITY TO YOUR MEDICAL DEVICE TUBING

- Monolithic Extrusion can impart antimicrobial properties to your new or existing medical device tubing and film design.
- This can be done to all standard medical device polymers used in the industry today.
- There are several methods of delivering antimicrobial properties and MI expertise can guide the selection process for you.
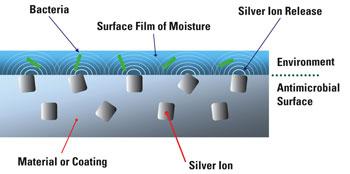
Hospitals are increasingly investing efforts and funds to control Hospital Acquired Infections (HAI’s)
Short and longer term - use catheter and devices can reduce biofilm bacterial colonies from developing on their device and reduce unwanted side effects associated with their device by including Antimicrobial technology into their device component design.
Broad-spectrum antimicrobial properties to address bacterium such as Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans and others can be achieved.
Adding Antimicrobial Technology to your medical device can reduce:
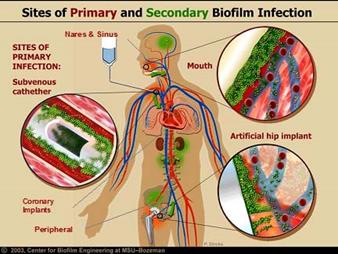
■ Surgical site infections.
■ Central line-associated bloodstream infections.
■ Ventilator-associated pneumonia.
■ Catheter-associated urinary tract infections.
Much of the technology used with polymer tubing already has FDA approval precedents. Many selections are non-toxic and are non-irritating to human skin and tissue. Long-term stability of the antibacterial action can also be achieved.
-
Create a market advantage in your next device development project with Antimicrobial Extrusion Technology from Monolithic Industries.
